RESEARCH AND DEVELOPMENT
The demand for new methods of treating tumors is growing, and Celluris is a pioneer in Brazil when it comes to the development of effective immunotherapies for the treatment of patients with cancer.
The company develops treatments using chimeric T-cell antigen receptor therapy, an innovation in the country and considered a more accurate treatment to fight cancer, for patients who no longer responded to other therapies.
IMUNOTHERAPY
The basic principle of immunotherapy is the use of the immune system itself to fight diseases, including cancer.
Immunotherapy oncological treatments can be performed in two ways:
1 by boosting the immune system in a way that it works more effectively in attacking cancer cells;
2 by introducing specific components of the immune system, such as proteins, so that they specifically and effectively attack cancer cells.
The adoption of immunotherapy varies according to the type of cancer and the methods can be used in conjunction with other types of treatment.
For some decades now, immunotherapy has been an important part of cancer treatment and new solutions have been studied and developed – capable of revolutionizing cancer treatment in the future. This is the case of the CAR-T treatment, an innovative treatment, developed in Brazil by Celuris.
CAR-T Technology
The chimeric T cell antigen receptor therapy, also known as CAR-T, is an immunotherapy that is innovating the treatment of hematological and solid tumors.
This new technology is considered less aggressive than conventional treatments, since it focuses only on cancer cells, not attacking healthy cells.
CAR-T technology works as follows:
a blood sample is taken from the patient. T-lymphocytes, cells from the immune system, are extracted from it;

in the laboratory, the lymphocyte DNA is genetically modified with CAR to recognize and attack cancer cells, in a more precise and effective way;
the modified T-lymphocytes are multiplied and reinserted in the patient, so that they can start to immune attack the tumor.
It is worth mentioning that the patient’s T-cells are modified according to the tumor the person is facing. Thus, the treatment is personalized, ensuring greater effectiveness.
Approved in 2017 in the United States and already available in Israel and Europe, this type of treatment is being tested in Brazil. Celluris is a pioneer in the country in research and development of CAR-T technology.
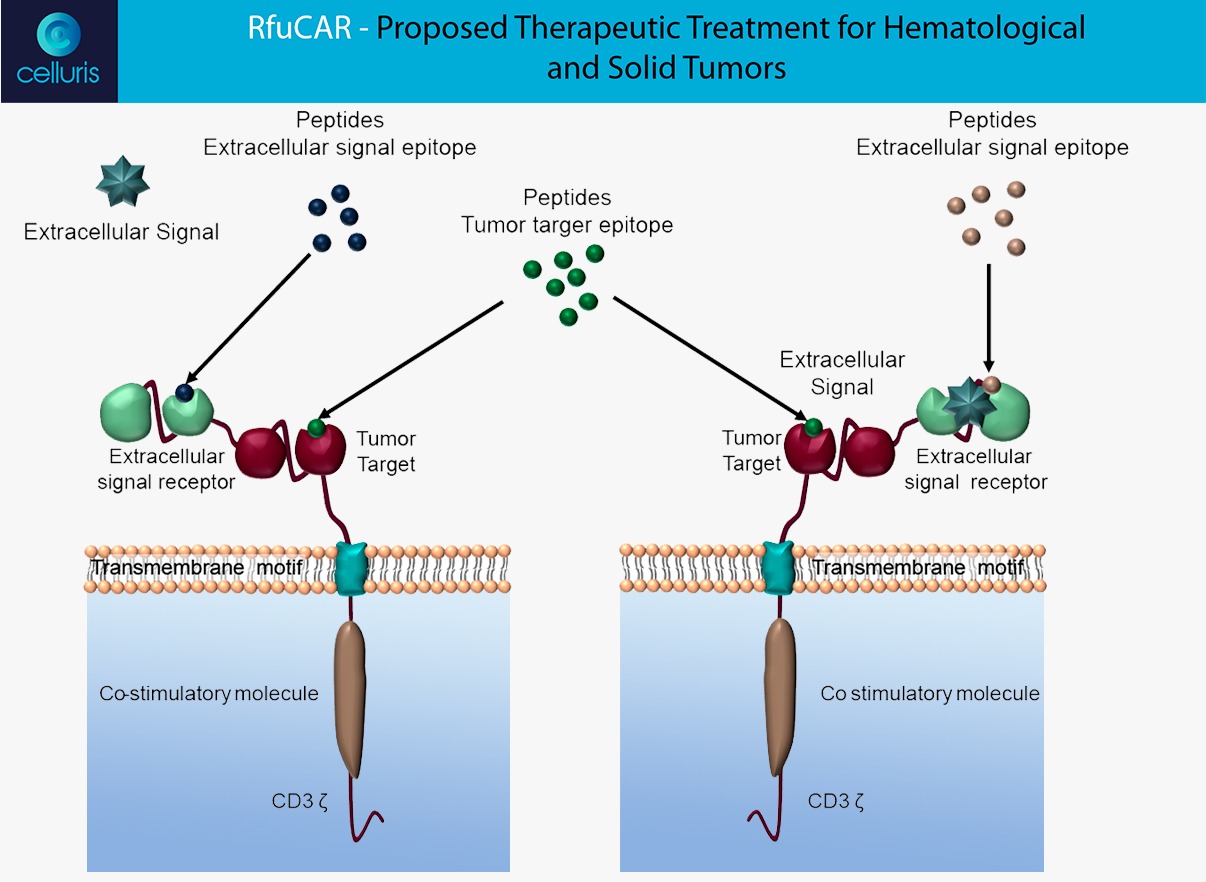
RfuCAR
The Celluris’ novel solution, named RfuCAR, was engineering in order to respond some challenges addressed by clinical trials around the world. RfuCAR is a bispecific in tandem receptor CAR, that is composed by an antigen binding domain and another domain that binds to an extracellular molecule from tumoral microenvironment. Therefore, our CAR-T solution can:
– Modulate the tumoral microenvironment by trapping an extracellular molecule, in order to reduce inhibitory signaling and to improve the CAR anti-tumoral function;
– This modulation is able to minimize the effects of CRS – Cytokine Release Syndrome and neurotoxicity, very common in patients treated with CAR-T therapy;
– Additionally, such mechanism is able to be switched-off by the administration of both receptor epitope peptides, constituting a regulatory safety switch;
– Ex vivo expansion protocols used intend to obtain a high grade purity and viability product, besides to stimulate T cells phenotype specific that increase the cytotoxicity and proliferation in vivo;
– In vitro and In vivo tests and models are being carried out to corroborate the advantages of our solution applications.
The figure above clarify the RfuCAR mechanisms: